‘Like Looking at a Miracle’: Baby Blossoms Thanks to Gene Therapy
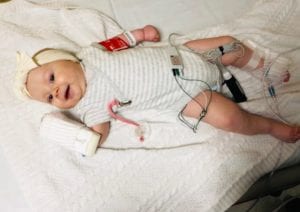 When Arabella Smygov was diagnosed with spinal muscular atrophy (SMA) type 1 at 3 months old, the first recommendation Dr. Fawn Leigh, a neurologist at Seattle Children’s, gave her parents, Sarah and Vitaliy, was to wait on searching for information about SMA online.
When Arabella Smygov was diagnosed with spinal muscular atrophy (SMA) type 1 at 3 months old, the first recommendation Dr. Fawn Leigh, a neurologist at Seattle Children’s, gave her parents, Sarah and Vitaliy, was to wait on searching for information about SMA online.
This is because up until a few years ago, SMA type 1 was a fatal diagnosis. Most of the information available online painted a bleak picture. Babies diagnosed with SMA type 1, the most severe and common form of the neurodegenerative disease, usually don’t survive beyond age 2 and if they do, they require full support for breathing from a ventilator.
Leigh had good reason for wanting her parents to have hope for Arabella’s future. Two treatments, including the first-ever drug approved for the condition by the U.S. Food and Drug Administration (FDA) in 2016 called Spinraza, and Zolgensma, a gene therapy approved by the drug agency this month, are rapidly changing the trajectory for children with Arabella’s condition.
 “I always remember back to when I had to offer my first SMA diagnosis,” Leigh said. “I was heartbroken to tell this young couple that we didn’t have anything for their baby. Now, we’re planning a future for these babies because we have not one, but two good treatment options.”
“I always remember back to when I had to offer my first SMA diagnosis,” Leigh said. “I was heartbroken to tell this young couple that we didn’t have anything for their baby. Now, we’re planning a future for these babies because we have not one, but two good treatment options.”
This post was originally written by Lindsay Kurs and originally appeared in the Seattle Children’s On The Pulse blog.





